RESEARCH
M. Capogrosso, T. Milekovic, G. Courtine
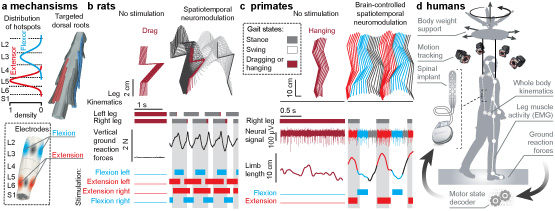
Figure 1. Translational spatiotemporal neuromodulation therapies. (a) Location of flexion and extension hotspots in the spinal cord, and 3D reconstruction of the dorsal root trajectories allowing to access these regions. Electrodes are placed over the dorsal aspect of the spinal cord to target flexion and extension hotspots. (b) Real-time control of spatiotemporal neuromodulation using movement feedback restored robust locomotion in paralyzed rats with complete injury. Adapted from Wenger et al. Nature Medicine 2016. (c) Brain-controlled spatiotemporal neuromodulation instantly restored weight-bearing locomotion of a paralyzed leg in monkeys. Adapted from Capogrosso et al. Nature 2016. Panel b and c show stick diagram decomposition and gait states of affected hindlimb, vertical ground reaction force (b only), neural signal of a selected electrode implanted in the leg area of the left motor cortex (c only), right limb length (c only), and the stimulation over the electrodes identified as the hotspots for left and right flexion and extension movements. D. Technological framework for application of spatiotemporal neuromodulation therapies in humans. View larger version of this figure (PDF).
A century of research in spinal cord physiology has demonstrated that the circuits embedded in the lumbar spinal cord of mammals can autonomously produce repetitive patterns of motor activity resembling locomotion [1]. After a spinal cord injury (SCI), however, the neural pathways carrying information between the brain and these spinal circuits, usually located below the injury, are partly or completely interrupted. While the lumbar circuits are intact, this interruption disrupts or abolishes volitional leg movements.
This understanding triggered a surge of interest for the development of neuromodulation interventions capable of re-activating these circuits after injury [2]. For example, the combination of serotonergic agonists and continuous epidural electrical stimulation (EES) applied to the lumbar spinal cord instantly restored a range of locomotor behaviours in paralyzed rats. Despite a complete SCI, the animals were able to walk across a range of speeds, to run, and even to locomote sideways [3].
These empirical observations prompted us to investigate the mechanisms underlying the production of locomotion during the delivery of EES. We were specifically interested in the interactions between the stimulation and spinal circuits. We found that EES engages specific neural components termed the muscle spindle feedback circuits. These circuits connect sensory receptors located in the muscles (muscle spindles) to motoneurons via monosynaptic and polysynaptic pathways. Therefore, the delivery of EES brings motoneurons to a state of enhanced excitability. Once this state is reached, residual descending inputs and/or sensory feedback can effectively recruit motoneurons, and thereby, can lead to the production of movements [4, 5]. This understanding allowed us to design evidence-based strategies to modulate specific muscle synergies. We conceived spinal electrode implants that targeted distinct muscle spindle feedback circuits located in specific dorsal roots in order to access restricted regions of the lumbar spinal cord. This spatial specificity supported the precise and independent modulation of flexion and extension muscle synergies with EES [6].
These combined results established the framework and building blocks for the development of a neuroprosthetic system capable of restoring locomotion in people with paraplegia. Indeed, preliminary clinical evaluations have shown the ability of continuous EES to elicit rhythmic activity resembling locomotor patterns after motor complete paralysis in humans [2]. However, the recovery of volitional locomotion requires the brain to control this activity. Brain–computer interface technologies [7] provide the tools to extract the intentions to move the leg from brain activity, and to link this information to EES protocols in order to promote these movements—and thus to re-establish a voluntary control of locomotion.
This scenario presents a series of challenges: (i) translate spinal cord stimulation protocols developed in rats to strategies that similarly modulate the human spinal cord; (ii) design algorithms and control systems capable of modulating EES protocols based on brain activity, and (iii) develop clinical-grade devices that acquire brain activity and deliver EES in real-time without the need of tethered electronics.
To address these challenges, we conducted a study in nonhuman primate models of SCI [8]. Compared to humans, nonhuman primates share comparable anatomical and functional features of the motor system [9], display similar cortical engagement during locomotion [10], show analogous recovery mechanisms from injury [11], and are compatible with medical-grade technologies [9]. In this work, we interfaced leg motor cortex activity with EES protocols to establish a wireless brain–spinal interface (BSI) that alleviated gait deficits after a SCI in two nonhuman primates.
Two Rhesus monkeys were implanted with a spinal cord stimulation system and a brain recording system. The spine-part of the BSI included a tailored spinal implant that was directly derived from the knowledge gained in rodents. The implant was connected to an implantable pulse generator (IPG) commonly used to deliver deep brain stimulation in clinical settings. However, a new firmware equipped the IPG with real-time triggering capabilities. The brain-part of the BSI involved an intra-cortical array of 96 electrodes inserted the leg area of the motor cortex. During recordings, the array was connected to a neurosensor mounted onto the animal’s skull. This device transmitted broadband neural data to an external computer that decoded the intended extension and flexion motor states based on the recorded motor cortex activity. The decoded motor states were then linked to EES protocols that promoted these extension and flexion movements of the paralyzed leg. Importantly, the wireless BSI allowed the monkeys to behave freely without any restrictions. As early as six days after a lateral under-hemisection of the spinal cord, the BSI restored plantar, weight-bearing movements of the paralyzed leg on a treadmill and overground. The stimulation protocols and resulting modulation of leg muscle activity were strikingly similar to those observed in rats, suggesting that the same protocols may also be effective in humans.
The devices integrated in the design of the wireless BSI have all been approved for research in humans, thus opening realistic perspectives for proof of concept studies in people with paraplegia. However, application of the BSI for restoring the bipedal locomotion of humans may require the integration of additional technologies. These include pharmaceutical interventions [3] to compensate for the interruption of serotonergic pathways; and robotic systems to help sustain balance. Moreover, the facilitation of leg movements underlying activities of daily living will require the development of task- dependent stimulation protocols and more elaborated decoding algorithms to adjust these protocols. Finally, future studies will have to determine whether the BSI may enhance neuroplasticity in conjunction with robot-assisted rehabilitation [12], and thus augment functional recovery after SCI.
References
- Sherrington, C., Flexion-reflex of the limb, crossed extension reflex, and reflex stepping and standing. J Physiol (Lond), 1910. 40: p. 28-121.
- Angeli, C.A., et al., Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain, 2014. 137(Pt 5): p. 1394-409.
- Courtine, G., et al., Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci, 2009. 12(10): p. 1333-1342.
- van den Brand, R., et al., Restoring Voluntary Control of Locomotion after Paralyzing Spinal Cord Injury. Science, 2012. 336(6085): p. 1182-1185.
- Edgerton, V.R., et al., Training locomotor networks. Brain research reviews, 2008. 57(1): p. 241-254.
- Wenger, N., et al., Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat Med, 2016. 22(2): p. 138-45.
- Shenoy, K.V. and J.M. Carmena, Combining Decoder Design and Neural Adaptation in Brain-Machine Interfaces. Neuron, 2014. 84(4): p. 665-680.
- Capogrosso, M., et al., A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature, 2016. 539(7628): p. 284-288.
- Courtine, G., et al., Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med, 2007. 13(5): p. 561-6.
- Lemon, R.N., Descending pathways in motor control. Annu Rev Neurosci, 2008. 31: p. 195-218.
- Friedli, L., et al., Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Sci Transl Med, 2015. 7(302): p. 302ra134.
- van den Brand, R., et al., Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science, 2012. 336(6085): p. 1182-5.
For Further Reading
- Capogrosso, M., et al., A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature, 2016. 539(7628): p. 284-288.
- Friedli, L., et al., Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Sci Transl Med, 2015. 7(302): p. 302ra134.
- van den Brand, R., et al., Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science, 2012. 336(6085): p. 1182-5.
Authors
 Marco Capogrosso: My main interest is the understanding of the neural control of movement with a focus on translational applications in motor disorders. My background in applied physics has strongly influenced my path since the beginning. Indeed, when I started my PhD program in Biomedical Engineering as a fellow of the Scuola Superiore Sant’Anna, in Pisa, I was looking neither for a purely theoretical research program nor purely experimental. My intention was to understand the basic interactions between neuromodulation technologies and sensorimotor circuit dynamics. I wanted to develop theoretical tools to support translation and bring them all the way down to the clinics to test whether my findings and ideas had any impact at all on actual clinical applications. After a PhD and a 3-yr post-doc program under the supervision of Prof. Silvestro Micera first and Prof. Grégoire Courtine later I am deeply convinced that a theoretical approach to translational neuroscience can have a significant impact on clinical applications. Indeed, I have used computational models to design and implement real-time neurotechnologies that I have tested in rats, non-human primates and humans. I have started with simple models of the peripheral nerve that I have slowly improved to complex neuro-biomechanical models of the spinal sensorimotor circuits, while at the same time performing animal experiments to test my models up to the implementation of real-time technologies able to restore sensation in human amputees and brain-controlled locomotion in non-human primates after spinal cord injury. Ultimately, I love science.
Marco Capogrosso: My main interest is the understanding of the neural control of movement with a focus on translational applications in motor disorders. My background in applied physics has strongly influenced my path since the beginning. Indeed, when I started my PhD program in Biomedical Engineering as a fellow of the Scuola Superiore Sant’Anna, in Pisa, I was looking neither for a purely theoretical research program nor purely experimental. My intention was to understand the basic interactions between neuromodulation technologies and sensorimotor circuit dynamics. I wanted to develop theoretical tools to support translation and bring them all the way down to the clinics to test whether my findings and ideas had any impact at all on actual clinical applications. After a PhD and a 3-yr post-doc program under the supervision of Prof. Silvestro Micera first and Prof. Grégoire Courtine later I am deeply convinced that a theoretical approach to translational neuroscience can have a significant impact on clinical applications. Indeed, I have used computational models to design and implement real-time neurotechnologies that I have tested in rats, non-human primates and humans. I have started with simple models of the peripheral nerve that I have slowly improved to complex neuro-biomechanical models of the spinal sensorimotor circuits, while at the same time performing animal experiments to test my models up to the implementation of real-time technologies able to restore sensation in human amputees and brain-controlled locomotion in non-human primates after spinal cord injury. Ultimately, I love science.
 Dr. Tomislav Milekovic (IEEE member) obtained his PhD from the Imperial Colleague London, worked as a postdoc at the Brown University, is currently a postdoc at the École Polytechnique Fédérale de Lausanne (EPFL), and will soon become a junior group leader at the University of Geneva. He investigates how neural activity, measured from the surface and within the cortex of primates, encodes movements, movement intentions and movement errors. He uses gained knowledge to develop and advance brain-computer interfaces, systems that allow subjects with paralysis to control external devices, like computers, robotic limbs or spinal cord stimulators, solely by their brain activity. He has won Morton Cure Paralysis Fund and Marie Curie fellowships, as well as grants from Swiss National Science Foundation, International Brain Research Organization and Swiss Parkinson’s Disease Foundation. He aims to transform the lives of people with paralysis by engineering brain- computer interfaces capable of both stable and complex control of prosthetic and assistive devices in order to restore their ability to move and communicate.
Dr. Tomislav Milekovic (IEEE member) obtained his PhD from the Imperial Colleague London, worked as a postdoc at the Brown University, is currently a postdoc at the École Polytechnique Fédérale de Lausanne (EPFL), and will soon become a junior group leader at the University of Geneva. He investigates how neural activity, measured from the surface and within the cortex of primates, encodes movements, movement intentions and movement errors. He uses gained knowledge to develop and advance brain-computer interfaces, systems that allow subjects with paralysis to control external devices, like computers, robotic limbs or spinal cord stimulators, solely by their brain activity. He has won Morton Cure Paralysis Fund and Marie Curie fellowships, as well as grants from Swiss National Science Foundation, International Brain Research Organization and Swiss Parkinson’s Disease Foundation. He aims to transform the lives of people with paralysis by engineering brain- computer interfaces capable of both stable and complex control of prosthetic and assistive devices in order to restore their ability to move and communicate.
 Grégoire Courtine was trained in Mathematics, Physics, and Neurosciences. He received his PhD degree in Experimental Medicine in France in 2003. After obtaining the Chancellor Award during his post-doctoral training at the University of California Los Angeles (UCLA), he established his own laboratory at the University of Zurich in 2008. In 2012, he became Associate Professor in the Center for Neuroprosthetics at the École Polytechnique Fédérale de Lausanne (EPFL). Over the past 15 years, Grégoire and his team have sought to develop new treatment paradigms for spinal cord injury. The results of this research were recognized in various high-profile publications, and discussed extensively in national and international media. In 2013, he was invited to share his scientific journey at TEDGlobal. In 2014, Grégoire launched his startup, G-Therapeutics, which aims to translate the medical and technological knowledge gained over the past 15 years into a treatment to accelerate and augment functional recovery after spinal cord injury.
Grégoire Courtine was trained in Mathematics, Physics, and Neurosciences. He received his PhD degree in Experimental Medicine in France in 2003. After obtaining the Chancellor Award during his post-doctoral training at the University of California Los Angeles (UCLA), he established his own laboratory at the University of Zurich in 2008. In 2012, he became Associate Professor in the Center for Neuroprosthetics at the École Polytechnique Fédérale de Lausanne (EPFL). Over the past 15 years, Grégoire and his team have sought to develop new treatment paradigms for spinal cord injury. The results of this research were recognized in various high-profile publications, and discussed extensively in national and international media. In 2013, he was invited to share his scientific journey at TEDGlobal. In 2014, Grégoire launched his startup, G-Therapeutics, which aims to translate the medical and technological knowledge gained over the past 15 years into a treatment to accelerate and augment functional recovery after spinal cord injury.


