RESEARCH
Junichi Ushiba, Keio University, Japan.
Note: This original study on shoulder movement restoration by using BMI in post-stroke hemiplegia was submitted to the Annual BCI Award 2017 hosted by g.tec., Austria, and was nominated among the top 12 1.
Following a stroke injury, the human brain initiates a dynamic process of functional remodeling in neural circuitry. However, this process typically halts when it reaches a chronic stage, approximately 6 months post-stroke 2. Previous studies have demonstrated that motor exercise supported by EEG-based brain–machine interface (BMI) facilitates the resumption and shaping of the neural remodeling process, probably through a variety of plasticity/learning mechanisms, such as Hebbian-like plasticity, reward-based reinforcement learning, and error-based learning 3. This occurs even in the non-plastic chronic stage, continuing to unmask remaining circuitry of the original corticospinal pathways in the ipsi-lesional hemisphere3,4. Randomized controlled trials suggest that the degree of functional recovery of finger and arm movements is relevant for actual clinical use 5,6.
However, the remodeling process of the ipsi-lesional M1 for finger motor recovery has been shown to impede shoulder movement recovery as the enlargement of motor areas associated with finger control can lead to erosion of motor areas responsible for shoulder movement 7,8. Also, not ipsilateral but contra-lesional M1 shows better control of paralyzed shoulder 9. Avoiding competitive reinnervation processes in the motor cortex may therefore help better motor improvement in the post-stroke. We tested a novel intervention with the aim of achieving targeted up-conditioning of contra-lesional corticospinal pathways by BMI for shoulder movement recovery as shoulder muscles are bi-hemispherically innervated.
Methods
BMI Intervention: A total of eight post-stroke patients with severe chronic hemiplegia were included in this case series pilot study. Clinical and neurophysiological measurements were performed 1 day pre- and post-intervention. One-hour BMI intervention training was conducted for seven consecutive days. Training consisted of 10 trials each repeated 10 times, for a total of 100 trials. Participants were asked to attempt shoulder flexion following a fixed repetitive time scheme, with 5 seconds of the resting-state and 5 seconds of attempting shoulder flexion. During the task period, the event-related desynchronization (ERD) of the sensorimotor rhythm (8–13 Hz) over the contra-lesional hemisphere (i.e., ipsilateral to the paralyzed arm) was observed. Visual feedbacks of ERD amplitude were provided for each task. When participants maintained ERD over 30% for 1 s during the motor attempting period, robotic assistance for shoulder flexion and neuromuscular electrical stimulation (13–17 mA, 100 Hz, 1 ms) to the anterior deltoid was applied as feedback (Fig. 1a).
The reason why we employed contra-lesional ERD is as follows. First, shoulder muscles are bihemispherically innervated, thereby some extent of potential motor path exists in the contra-lesional side. Second, shoulder motor areas in the ipsilesional side are often eroded by enlargement of finger motor area, following intensive finger training. Third, previous clinical studies suggest the importance of the up-conditioning of contra-lesional motor area for functional shoulder motor recovery 10. Because BMI technology can target the intended cortical area by determination of electrode placement and EEG feature, the present study tested its impact on shoulder motor recovery.
Powered exoskeleton for shoulder elevation: Since paralyzed muscles around shoulder loosen their tensions, shoulder joint kinematics in post-stroke severe hemiplegia is characterized as potential subluxation and pain. To prevent shoulder impingement, where shoulders rotator cuff tendons are trapped and compressed during shoulder movements causing injury to the shoulder tendons and bursa, we newly designed a powered exoskeleton as follows. First, the mechanical torque is applied directly to the shoulder joint, and not a push-up the limb endpoint, since push-up the limb endpoint is a clinically known movement of shoulder impingement. Second, the robotic parts are compliant and softly attached to the paralyzed limb. Thanks to this mechanism, hypertension to the shoulder joint was safely dodged by the arm slipped during robot movement. Third, joint torque is applied by the compliant pneumonic drivers that exhibits a high degree of flexibility and stability of shoulder elevation movement which to a large extend is ensured by local properties of the musculo-skeletal system and reflexes. Due to this mechanism, net torque around the joint is smoothly maintained the target level, even physical compliance is deviated from the initial by the increased reflexes or muscle stiffness. Fourth, robotic movement mimic pre-registered therapist’s movement. Spasticity is a function of limb position and motion speed, and is varied among patients. Thus, playback of the locus of therapist’s manual torque in the physical exercise is best to avoid spasticity risk during BMI exercise. It is also noted here that the powered exoskeleton applies neuromuscular electrical stimulation to the anterior deltoid muscle via a pair of surface electrodes in order to induce muscle spindle activities as a somatosensory feedback signal to the brain.
Assessment: A 128-channel whole-head EEG was used to assess the hemispheric lateralization of ERD. The laterality index value was computed using the following formula, where cERD and iERD are the values of contra- and ipsi-lesional ERD, respectively.
Laterality index (LI)=((cERD-iERD)/(|cERD|+|iERD| ))
In five of the participants, MEP of the anterior deltoid was measured during shoulder flexion of 20% isometric maximum voluntary contraction with paretic side. TMS was applied over the contra-lesional M1 at 110% active motor threshold. Three other patients were excluded from TMS assessment for avoiding risk of seizure. The arm section of the Fugl-Meyer Assessment (FMA) was used for primary outcome measure. Minimal clinically important difference (MCID) value was determined to be a 10% increase from the initial FMA score (i.e., 1.68), as previously defined by the critical review of post-stroke motor recovery researches [11].
3. Results
All participants finished the BMI interventions with no reported or obvious adverse effects. The group analysis of hemispheric lateralization of ERD using LI value (Fig. 1b) demonstrate that contra-lesional ERD became dominant following BMI interventions (p < 0.01). Following the intervention, three participants (#1, 4, 6) showed increased MEPs, and one (#8) showed reappearance of MEP (not shown). All patients demonstrated improved FMA, and the average increase of FMA across participants was 6.63 ± 3.16. They all exceeded values of MCID (representative data is shown at Fig. 1c).
4. Discussion
Targeted up-conditioning of contra-lesional corticospinal pathways: Results from this study demonstrate lateralized ERD (increased contra-lesional ERD), and an appearance of TMS evoked potential in the paralyzed shoulder muscles. These findings significantly indicate the targeted up-conditioning of the contra-lesional corticospinal pathways, including M1, spinal motoneuron pools, and associated skeletal muscles. Resulting gains in active range-of-motion of the affected arm, with flexion movement exceeding the standardized MCID suggest that the targeted up-conditioning of contra-lesional corticospinal pathways satisfied the required amount of kinematic improvement for shoulder flexion movement.
Clinical impact: Impairment of shoulder elevation in post-stroke hemiplegia is a debilitating condition with no evidence-based, accessible treatment. Shoulder elevation is a crucial requirement for fundamental ADL, such as movements for dressing, grooming, eating, and drinking. For the first time, our novel BMI interventions were successful in the promotion of shoulder flexion movements in post-stroke patients with severe chronic hemiplegia who were unable to perform ADL. Participants demonstrated significant motor recovery beyond the standardized MCID, suggesting the strong potential of this as an engaging treatment with no currently identified side-effects at present.
Challenges of BMI: Our findings suggest the potential value of direct contra-lesional M1 control of paralyzed shoulder movement as a physiotherapy intervention for upper-extremity control in post-stroke patients with severe chronic hemiplegia. Movement support aided by online decoding of contra-lesional M1 activity along with exoskeleton shoulder robotics that mimicked therapists’ intervention is a novel, noninvasive, clinically effective treatment with no currently identified side-effects. It is probable that the facilitation of contra-lesional M1 remodeling by BMI may avoid competition of reinnervation processes between shoulder and finger areas in the ipsi-lesional motor cortex. Among neurorehabilitation techniques, there are no current, existing substitute interventions for such direct manipulation and facilitation of the brain remodeling process for volitional motor control.
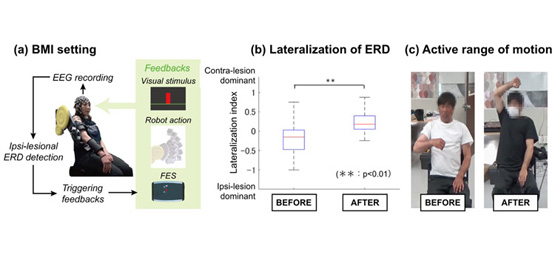
Figure 1. Experimental settings and associated outcomes of BMI interventions for arm flexion exercise
(a) Schematic of BMI interventions. 128-channel EEG cap was placed over the scalp, and a laplacian derivate of EEG was used to decode the ERD amplitude focally around contra-lesional M1. Visual feedback of the decoded ERD was given during the task, and robotic assistance of shoulder flexion and functional electrical stimulation to the anterior deltoid were applied as feedbacks when ERD exceeded the predetermined threshold of 30%. (b) Lateralization of ERD. The pooled data indicated ERD shift to the contra-lesional side after BMI intervention. (c) Active range of motion in paralyzed shoulder joint. The patients demonstrated improved arm flexion function exceeding over the standardized MCID.
5. Conclusion
The results from this study demonstrate the safety and efficacy of direct brain control of advanced exoskeleton robotics as a novel physiotherapeutic intervention. Electrophysiological assays support the evidence of targeted up-conditioning of contra-lesional corticospinal pathways in post-stroke patients with severe chronic hemiplegia. Movement support aided by online decoding of contra-lesional M1 activity and exoskeleton shoulder robotics that mimicked therapist’ intervention is a noninvasive, nonpharmacological, and clinically engaging treatment with no presently identified side-effects. Among rehabilitation robotics, there are no existing substitute means for the present therapeutic objectives, in terms of motor severity. EEG-based BMI may facilitate the targeted up-conditioning of contra-lesional corticospinal pathways, resulting in clinically relevant functional recovery of movement. Further study is expected to be conducted to evaluate the effectiveness and safety of the proposed BMI exercise for shoulder motor recovery, under randomized controlled multicenter trials on large patient groups.
References
- Takasaki K, Liu F, Hiramato M, Okuyama K, Kawakami M, Mizuno K, Kasuga S, Noda T, Morimoto J, Fujiwara T, Ushiba J, Liu M. The Annual BCI Award 2017 Top 12 nominees: Targeted up-conditioning of contralesional corticospinal pathways promotes motor recovery in poststroke patients with severe chronic hemiplegia. http://www.bci-award.com/2017/
- Nakayama H et al. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 75(4):394-8, 1994.
- Ushiba J & Soekadar S. Brain-machine interfaces for rehabilitation of poststroke hemiplegia. Prog Brain Res 228:163-83, 2016. DOI: 10.1016/bs.pbr.2016.04.020
- Shindo et al. Effects of neurofeedback training with an electroencephalogram-based brain-computer interface for hand paralysis in patients with chronic stroke: a preliminary case series study. J Rehabil Med 43(10):951-57, 2011. doi: 10.2340/16501977-0859.
- Ramos-Murguialday A et al. Brain-Machine-Interface in Chronic Stroke Rehabilitation: A Controlled Study. Ann Neurol 74(1):100-8, 2013. DOI: 10.1002/ana.23879
- Pichiorri F et al. Brain–computer interface boosts motor imagery practice during stroke recovery. Ann Neurol 77:851-65, 2015. DOI: 10.1002/ana.24390
- Chen R, Cohen L & Hallett M. Role of the ipsilateral motor cortex in voluntary movement. Can J Neurol Sci 24(4):284-91, 1997. DOI: 10.1017/S0317167100032947
- Muellbacher W et al. Improving Hand Function in Chronic Stroke. JAMA Neurology 59(8):1278-1282, 2002. DOI: 10.1001/archneur.59.8.1278
- Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional Hemisphere Control of the Proximal Paretic Upper Limb following Stroke. Cereb Cortex 22(11): 2662–2671, 2012. DOI: 10.1093/cercor/bhr344
- Schwerin S et al. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res 185(39):509-519, 2007. DOI: 10.1007/s00221-007-1169-8
- Gladstone D et al. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 16(3):232-40, 2002. DOI: 10.1177/154596802401105171
About the author:

Junichi Ushiba is an Associate Professor at the Department of Biosciences and Informatics, Faculty of Science and Technology, Keio University, Japan. He is also appointed as a Principal Investigator at the Keio Institute of Pure and Applied Sciences (KiPAS). He holds a Ph.D. in Engineering from Keio University in 2004. He is an executive board member of the Clinical BMI Society, and a chair of the Real-Time Functional Imaging and Neurofeedback Conference 2017. He received the Young Scientists’ Prize by the Ministry of Education, Culture, Sports, Science and Technology, Japan in 2015. His research focuses on remodeling of brain functions aided by brain-machine interfaces (BMI). In collaboration with Panasonic, he has recently been managing product development of BMI for functional recovery from post-stroke hemiplegia.


