Ye Tian1,2, Cunkai Zhou1, Kuikui Zhang1, Huiran Yang3, Zhaohan Chen3, Zhitao Zhou2,3, Xiaoling Wei2,3, Tiger H. Tao1,2,3,4,5,6,7,8,9,*, Liuyang Sun1,2,3,*
1 2020 X-Lab, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Shanghai 200050, China
2 School of Graduate Study, University of Chinese Academy of Sciences, Beijing 100049, China
3 State Key Laboratory of Transducer Technology, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Shanghai 200050, China
4 Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
5 School of Physical Science and Technology, ShanghaiTech University, Shanghai 200031, China
6 Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai 200031, China
7 Neuroxess Co., Ltd. (Jiangxi), Nanchang, Jiangxi 330029, China
8 Guangdong Institute of Intelligence Science and Technology, Hengqin, Zhuhai, Guangdong 519031, China
9 Tianqiao and Chrissy Chen Institute for Translational Research, Shanghai, China.
* Corresponding Author: tiger@mail.sim.ac.cn, liuyang.sun@mail.sim.ac.cn
Abstract
Implantable flexible neural probes have been demonstrated bridging the mechanical mismatch between invasive probes and brain tissues, minimizing footprint in brain, and chronic biocompatibility [1]. However, conventional needle-shaped flexible neural probes reported before have recording sites distributed vertically along a relatively narrow shank [2], which limits the lateral range in which the probes may record neural signals. Although designs with more probe shanks expand the lateral detectable range, the high implantation density reflects in increased tissue damage and surgery complexity. In this work, we developed a flexible neural probe by novel Christmas-tree structure, which has branches that are foldable along the shank by temporary encapsulation before implantation and self-stretchable after the encapsulation dissolves after implantation. The probe we developed affords increased lateral sensing range without causing extra brain tissue damage.
Main
Recently, flexible neural probes have been attracting great attentions due to their mechanical matching with the brain and outstanding chronic performance. To minimize the bending stiffness and footprint in the brain, the flexible probe usually has a long yet narrow shank along which the electrode contacts are distributed. An electrode contact could capture intercellular action potential from neurons within only about 20 micrometers around the electrode [3]. Therefore, a typical flexible probe could detect neurons longitudinally along its shank but lack the accessibility to neuron laterally away from the shank [4,5]. While spikes from a population of neurons distributed laterally could be captured by multiple flexible probes implanted, such a method inevitably introduces more footprints during surgery which cause more tissue damage, because the flexibility of neural probes naturally precludes independent penetration through the brain [6], and generally flexible probes are attached to rigid shuttle devices. In our work, we used tungsten wires to facilitate insertion. In addition, it is challenging to precisely insert multiple probes within a small area at the scale of hundreds by hundreds micrometers.
To expand the lateral detecting range of single shank probe, we designed and fabricated Christmas-tree-shaped neural probes, which remain one shank configuration and have electrodes on side-branches out of the main shank that could expand the sensing region at the direction perpendicular to the shank (Fig. 1a). Before implantation, the branches will be folded and fixed toward the shank of the probe without increasing lateral dimension by using materials such as silk or polyethylene glycol (PEG) as temporary encapsulation (Fig. 1b). In this work, we chose PEG because it is commonly used for animal experiments. After insertion and dissolution of encapsulation, the side-branches will unfold due to stress built up at the junction between shank and branches, and increase the lateral range where the electrodes reach. Since the size of probe is restricted by encapsulation, no extra footprint will be caused in surgery, as illustrated in Fig. 1c. The width and thickness of the branches are both minimized so little damage will be made during unfolding process.
We started with demonstrating the self-stretching of the Christmas-tree-shaped probes. Fig. 2a shows the structure of the flexible probe. It was fabricated by polyimide with a total thickness of 5 μm, and had 20 electrodes distributed on the main shank and 12 electrodes on the tip of each branch dispersed on both sides of the shank. We used molten PEG for the strongest surface tension and moderate period of time for assembly. After attaching neural probes to tungsten wires, molten PEG was dripped onto tip of the probe to form a droplet, and branches were attached to it due to surface tension. Before encapsulation completely cured, we transferred part of the droplet by pipette to reduce its size and thus improve the degree of folding of branches (Fig. 2b). For in vitro self-stretching experiment of our probes, we used 0.8% agar to mimic the mechanical property of brain tissue. Due to the stress built up at junctions between branches and the main shank (Fig. 2d), the branches stretched out naturally as encapsulation dissolved right after we penetrated the probe into agar, as shown in Fig. 2c. Therefore, the encapsulated Christmas-tree-shaped flexible probes could be delivered into the brain tissue with minimized invasiveness, and expand laterally after insertion to cover more brain regions for recording.
Next, we analyzed the self-stretching ability of branched flexible probes. Assuming that PEG droplets are totally wrapped by the shank and branches, the probe forms a circular bending structure and stress is distributed along the branches, as displayed in Fig. 2d. In that situation, the outer surface and inner surface of the bended polyimide undergo tensile and compressive strain respectively. The stress is determined by radius of curvature and film thickness [7]. Tensile stress and compressive stress are expressed in Equation 1 and 2 respectively,
 where t is film thickness, R is radius of curvature and E is Young’s modulus. As shown in Fig. 2d, the stress increases with the increase of polyimide film thickness. To detect further neuron from the probe, longer branches are required and the stress increases. These equations and results provide guidance for the thickness of the probe to achieve larger lateral detectable range.
where t is film thickness, R is radius of curvature and E is Young’s modulus. As shown in Fig. 2d, the stress increases with the increase of polyimide film thickness. To detect further neuron from the probe, longer branches are required and the stress increases. These equations and results provide guidance for the thickness of the probe to achieve larger lateral detectable range.
In conclusion, we demonstrated a novel Christmas-tree-shaped flexible neural probe with branch structures that could be folded before surgery for minimized tissue damage and self-stretch after implantation for expanded lateral detectable range. We believe this probe will be a proper solution to the problem of trade-off between lateral sensing range and tissue damage of flexible neural probes.
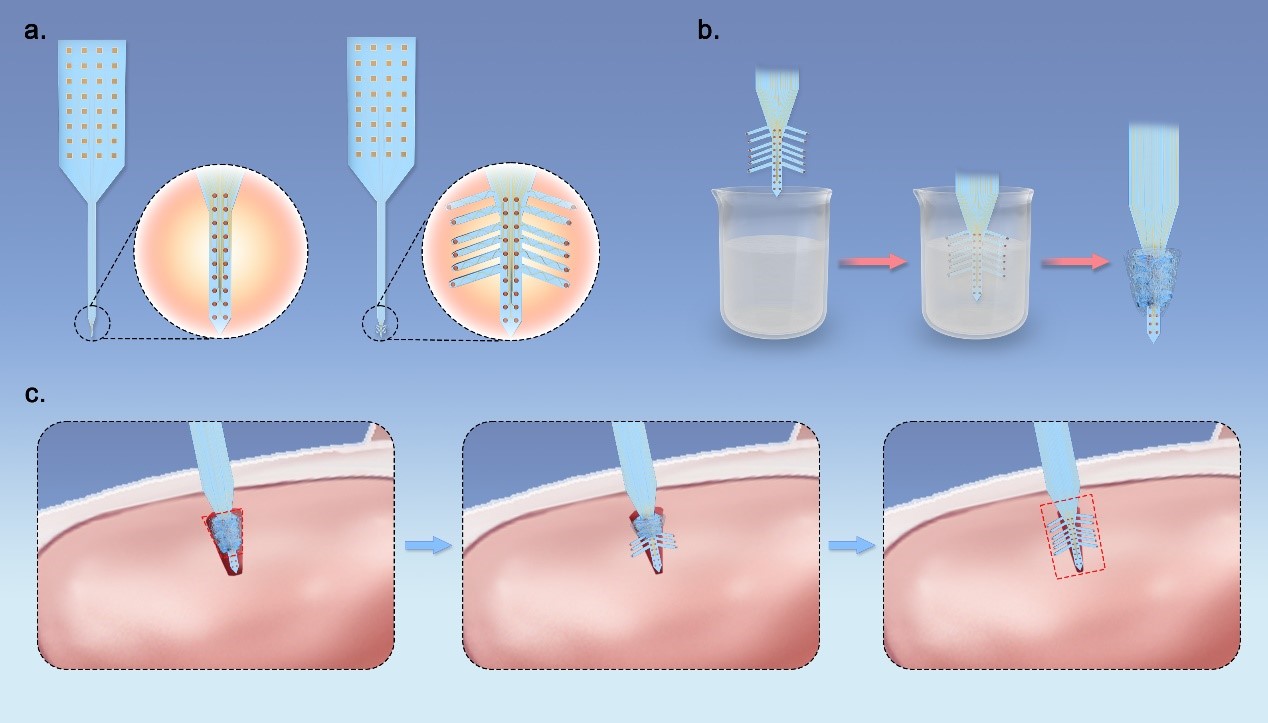
Fig. 1. (a) Comparison between conventional one-shank flexible neural probe and Christmas-tree-shaped flexible probe. (b) Process of temporary encapsulation before surgery. (c) Schematic of self-stretching of branch structure for expanded lateral sensing range after implantation.
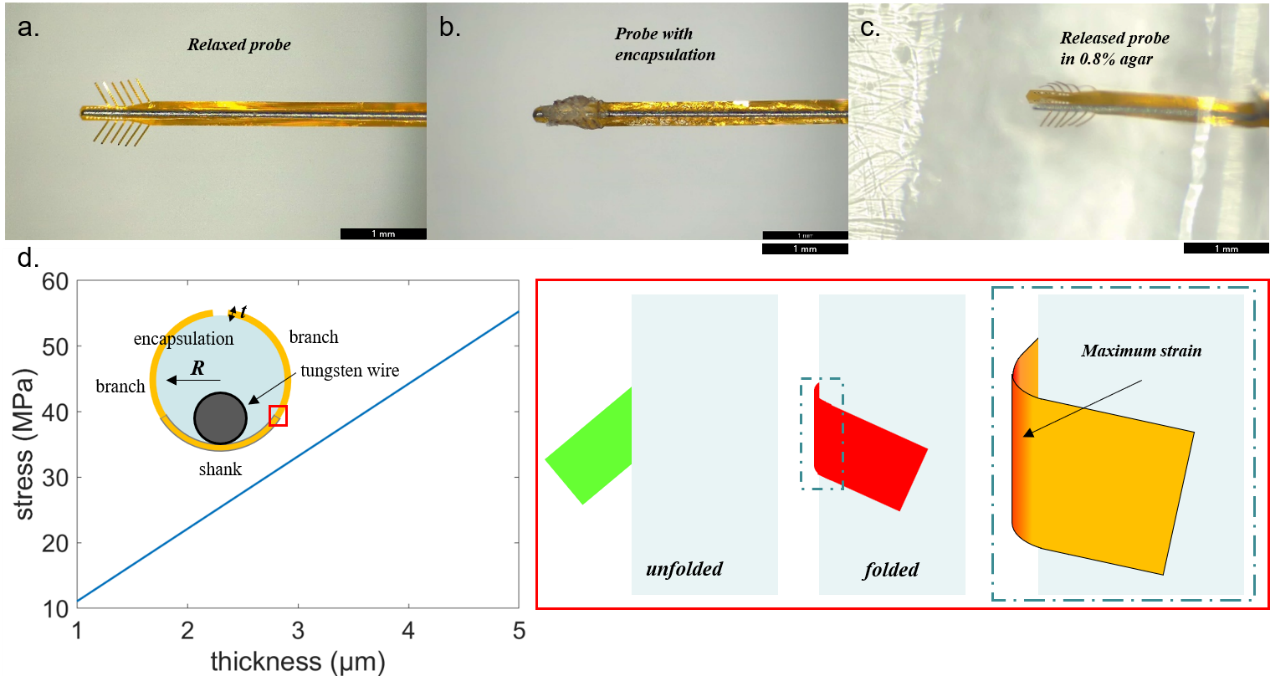
Fig. 2. (a) Relaxed Christmas-tree-shaped flexible probe with tungsten wire as shuttle device. (b) Christmas-tree-shaped probe with folded branches after encapsulating. (c) Released probe in 0.8% agar. (d) Schematic of polyimide bending model and plot of relationship between polyimide film thickness and stress.
Acknowledgement
This work was partially supported by the National Key R & D Program of China (Grant Nos. 2021ZD0201600, 2019YFA0905200, 2021YFC2501500,2021YFF1200700, 2022ZD0209300, 2022ZD0212300), National Natural Science Foundation of China (Grant No. 61974154), Key Research Program of Frontier Sciences, CAS (Grant No. ZDBS-LY-JSC024), Shanghai Pilot Program for Basic Research – Chinese Academy of Science, Shanghai Branch (Grant No. JCYJ-SHFY-2022-01), Shanghai Municipal Science and Technology Major Project (Grant No. 2021SHZDZX), CAS Pioneer Hundred Talents Program, Shanghai Pujiang Program (Grant Nos. 19PJ1410900, 21PJ1415100), the Science and Technology Commission Foundation of Shanghai (No. 21JM0010200), Shanghai Rising-Star Program (Grant No. 22QA1410900), the Innovative Research Team of High-level Local Universities in Shanghai, the Jiangxi Province 03 Special Project and 5G Project ( Grant No. 20212ABC03W07), Fund for Central Government in Guidance of Local Science and Technology Development (Grant No. 20201ZDE04013), Special Fund for Science and Technology Innovation Strategy of Guangdong Province (Grant Nos. 2021B0909060002, 2021B0909050004).
References
[1] L. J. Tang et al. Progress in research of flexible MEMS microelectrodes for neural interface. Micromachines (Basel). 2017;8(9):1–18. Doi: 10.3390/mi8090281 [2] J. Viventi et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 2011;14(12):1599–1605. Doi: 10.1038/nn.2973 [3] D. Kleinfeld et al. Can One Concurrently Record Electrical Spikes from Every Neuron in a Mammalian Brain? Neuron. 2019;103(6):1005–1015. Doi: 10.1016/j.neuron.2019.08.011 [4] X. Wei et al. Nanofabricated Ultraflexible Electrode Arrays for High-Density Intracortical Recording. Advanced Science. 2018;5(6):1700625. Doi: 10.1002/advs.201700625 [5] M. Du et al. Simultaneous surface and depth neural activity recording with graphene transistor-based dual-modality probes. Biosens Bioelectron. 2018;105:109–115. Doi: 10.1016/j.bios.2018.01.027 [6] L. Luan et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Science Advances. 2017;3(2):e1601966. Doi: 10.1126/sciadv.1601966 [7] D. Kong et al. High Cycle-life Shape Memory Polymer at High Temperature. Sci Rep. 2016;6:33610. Doi: 10.1038/srep33610
