Jihun Lee1, Ahhyoung Lee1, Vincent Leung2, Farah Laiwalla1, Arto Nurmikko1,3
1 School of Engineering, Brown University, Providence, RI, USA
2 Electrical and Computer Engineering, Baylor University, Waco, TX, USA
3 Carney Institute for Brain Science, Brown University, Providence, RI, USA
The concept of brain circuits computing as an extended network, composed of billions of neurons represents a contemporary view which is exploited in research of brain-machine interfaces (BMI). Population dynamics recorded from ensembles of neurons have been dominated by intracortical silicon-based microelectrode arrays (MEA), monolithic ‘beds of needles’, wired to external signal processing electronics. The work has deepened our understanding of underlying functional principles especially of the motor cortex as a network, leading to first clinical trials of human BMIs [1-4]. The importance of computational techniques in neural decoding in this highly undersampled circumstance is demonstrated in the example study: e.g. recent work by the Stanford group where pattern recognition of spiking neural population has demonstrated a BMI hand writing-to-text capability [5]. A forward-looking question is about the type of neural recording device technologies which are scalable and able to access a much larger number of neurons for decoding complex motor, sensory, and perhaps even cognitive tasks.
To attain such goals, many researchers are today relying on advanced semiconductor technologies to design implantable neural probes with a large number of channels. In one approach, a monolithic silicon blade integrating CMOS circuits with multiple multiplexed electrodes sites allows scaling of the channel count in the vertical cortical dimension [6]. Advances have also been made to build wireless neural implants at various levels of active circuit integration in anticipation of BMIs for mobile use [7-9].
A contrasting approach envisions ensembles of active recording microchips (Fig. 1) implanted as a spatially distributed sensor network, to capture cortical processes across multiple functional areas. In such a sensor network, each individual implant needs to operate in a fully autonomous manner by harvesting wireless energy, recording neural signals, and transmitting data. The concept of a neural sensor network has been proposed a decade ago and followed by several early proof-of-concept experimental studies [10, 11]. The work has mainly focused on developing the internal microelectronics for individual free-floating sensors and demonstrated in animal models as single devices, not addressing the challenges of wireless data networking, a critical aspect in building a large-scale sensor network. Further, the volume of the individual neural recording microchips has remained rather large (> 1mm3) in terms of minimizing neural tissue displacement for an intracortical implant.
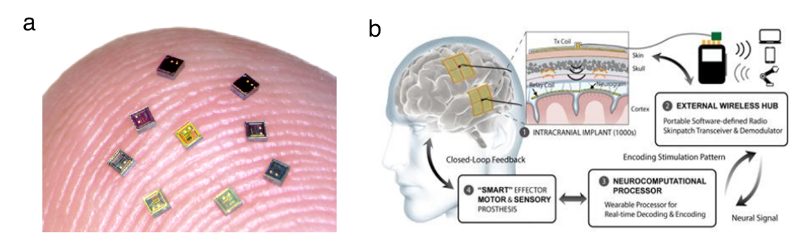
Figure 1. (a) Microphotograph of “Neurograin” chips on the tip of the finger. Photo credit: Jihun Lee (b) Concept of spatially distributed neurograin network with an external wearable wireless hub for a closed-loop BCI [13].
In ongoing work at our laboratories, we have designed and microfabricated sub-mm sized microchips for implantable use, “neurograins”, in which ultralow-power amplifier analog and digital circuits such as A/D converter and state machines which co-habit RF circuits, the latter designed to leverage wireless techniques previously developed in the telecommunication industry [12-19]. Our approach fuses together two wireless functions on chip, adapted to near-field use, radio-frequency identification (RFID) and specific multiple access telecom protocols, respectively. Each neurograin, the size of a grain of salt, operates as a special purpose active RFID tag, harvesting RF wireless energy near 1 GHz and transmitting recorded neural data through RF backscattering by modulating an on-chip coil impedance. This method eliminates the need for a separate transmitter and results in a small volume (~0.1 mm3).
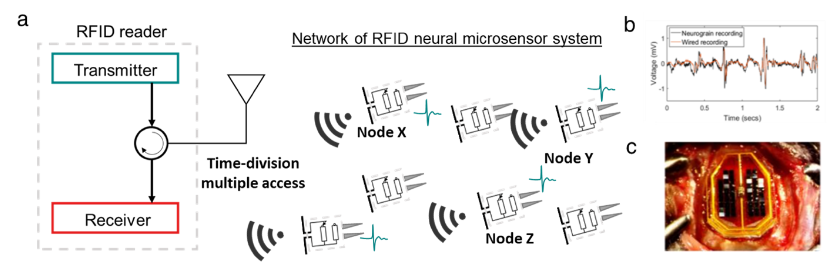
Figure 2. (a) The schematic for the implementation of time-division multiple access and RFID on neural implants. (b) Recording on a proxy-neural signal using neurograin wireless microchip. (c) In-vivo experiment using distributed 48 neurograins.
To enable communication from potentially large ensembles of wireless neurograins as illustrated in Figure 2a, on-board circuits have custom designs to enable programming for a specific multiple access protocol. Nonetheless, implementing a scheme such as time-division multiple access (TDMA) is challenging, mainly due to clock variance across an ensemble from the inhomogeneous magnetic field generated by the external source. This near-far problem results in varying on-chip voltage levels as well as clock frequencies. We have developed a custom TDMA protocol, a “call-and-response TDMA” whereby each microsensor has a unique device ID and transmits its data only when it detects its ID address from incoming RF commands (“downlink”). By doing that, we can sequentially call each microdevice at a specific time to avoid data packet collisions and minimize error rates in communicating with multiple nodes. In another variant of the TDMA protocol, we designed an autonomous TDMA scheme where each tag transmits its signal for a short period of 100 microseconds at a pseudorandom time to avoid packet collisions statistically. The latter has a limitation in the scalability while benefiting from inherent simplicity and requires minimal resources.
As an illustration of performance, Figure 2b shows an example of wireless signals recorded in a brain mimicking saline environment, where the externally induced electrical potentials display (here) a close matching in the frequency spectrum to the signal acquired by conventional wired probes. After post-processing the microchips into implants by fabricating on-chip electrodes and hermetic sealing [20], we performed in-vivo experiments in anesthetized rats to monitor neuronal signals. Separately designed neurograins have also been produced for neural electrical stimulation and induce neural responses through intracortical electrical stimulations. Further details experiments can be found in [12].
Encouraged by these results, our team is currently expanding the performance of the wireless neural microchips to include the recording of many different types of neural signals, while advancing the telecommunication approaches to scale the network capabilities to a high number of channels, up to many thousands of nodes. We believe that this kind of wireless sensor network would be applicable for other biomedical applications as well in the sense that it allows distributed sensing, low power, small volume, and high scalability.
Acknowledgments
This study has been collaborative research. We would like to acknowledge Farah Laiwalla, Jiannan Huang, Peter Asbeck, Patrick P. Mercier, Stephen Shellhammer, Lawrence Larson, and for their essential contributions to this work.
References
[1] Georgopoulos, A. P., Schwartz, A. B., & Kettner, R. E. (1986). Neuronal population coding of movement direction. Science, 233(4771), 1416-1419.
[2] Downey, J. E., Weiss, J. M., Muelling, K., Venkatraman, A., Valois, J. S., Hebert, M., … & Collinger, J. L. (2016). Blending of brain-machine interface and vision-guided autonomous robotics improves neuroprosthetic arm performance during grasping. Journal of neuroengineering and rehabilitation, 13(1), 1- 12.
[3] Hochberg, L. R., Serruya, M. D., Friehs, G. M., Mukand, J. A., Saleh, M., Caplan, A. H., … & Donoghue, J. P. (2006). Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature, 442(7099), 164-171.
[4] Heelan, C., Lee, J., O’Shea, R., Lynch, L., Brandman, D. M., Truccolo, W., & Nurmikko, A. V. (2019). Decoding speech from spike-based neural population recordings in secondary auditory cortex of non-human primates. Communications biology, 2(1), 1-12.
[5] Willett, F. R., Avansino, D. T., Hochberg, L. R., Henderson, J. M., & Shenoy, K. V. (2021). High-performance brain-to-text communication via handwriting. Nature, 593(7858), 249-254.
[6] Steinmetz, N. A., Aydin, C., Lebedeva, A., Okun, M., Pachitariu, M., Bauza, M., … & Harris, T. D. (2021). Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. Science, 372(6539), eabf4588.
[7] Musk, E. (2019). An integrated brain-machine interface platform with thousands of channels. Journal of medical Internet research, 21(10), e16194.
[8] Borton, D. A., Yin, M., Aceros, J., & Nurmikko, A. (2013). An implantable wireless neural interface for recording cortical circuit dynamics in moving primates. Journal of neural engineering, 10(2), 026010.
[9] Song, Y. K., Borton, D. A., Park, S., Patterson, W. R., Bull, C. W., Laiwalla, F., … & Nurmikko, A. V. (2009). Active microelectronic neurosensor arrays for implantable brain communication interfaces. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 17(4), 339-345.
[10] Seo, D., Carmena, J. M., Rabaey, J. M., Maharbiz, M. M., & Alon, E. (2015). Model validation of untethered, ultrasonic neural dust motes for cortical recording. Journal of neuroscience methods, 244, 114-122.
[11] Jia, Y., Mirbozorgi, S. A., Lee, B., Khan, W., Madi, F., Inan, O. T., … & Ghovanloo, M. (2019). A mm-sized free-floating wirelessly powered implantable optical stimulation device. IEEE transactions on biomedical circuits and systems, 13(4), 608-618.
[12] Lee, J., Leung, V., Lee, A. H., Huang, J., Asbeck, P., Mercier, P. P., … & Nurmikko, A. (2021). Neural recording and stimulation using wireless networks of microimplants. Nature Electronics, 4(8), 604-614.
[13] Lee, J., Mok, E., Huang, J., Cui, L., Lee, A. H., Leung, V., … & Laiwalla, F. (2019, March). An implantable wireless network of distributed microscale sensors for neural applications. In 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER) (pp. 871-874). IEEE.
[14] Lee, Jihun, et al. “Wireless power and data link for ensembles of sub-mm scale implantable sensors near 1GHz.” 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS). IEEE, 2018.
[15] Leung, V. W., Cui, L., Alluri, S., Lee, J., Huang, J., Mok, E., … & Laiwalla, F. (2019, April). Distributed microscale brain implants with wireless power transfer and Mbps bi-directional networked communications. In 2019 IEEE Custom Integrated Circuits Conference (CICC) (pp. 1-4). IEEE.
[16] Laiwalla, F., Lee, J., Lee, A. H., Mok, E., Leung, V., Shellhammer, S., … & Nurmikko, A. (2019, July). A distributed wireless network of implantable sub-mm cortical microstimulators for brain-computer interfaces. In 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 6876-6879). IEEE.
[17] Leung, V. W., Lee, J., Li, S., Yu, S., Kilfovle, C., Larson, L., … & Laiwalla, F. (2018, September). A CMOS distributed sensor system for high-density wireless neural implants for brain-machine interfaces. In ESSCIRC 2018-IEEE 44th European Solid State Circuits Conference (ESSCIRC) (pp. 230-233). IEEE.
[18] Lee, A. H., Lee, J., Jang, J., Nurmikko, A., & Song, Y. K. (2021). Wireless addressable cortical microstimulators powered by near-infrared harvesting. ACS sensors, 6(7), 2728-2737.
[19] Huang, J., Laiwalla, F., Lee, J., Cui, L., Leung, V., Nurmikko, A., & Mercier, P. P. (2018). A 0.01-mm 2 mostly digital capacitor-less AFE for distributed autonomous neural sensor nodes. IEEE Solid-State Circuits Letters, 1(7), 162-165.
[20] Lee, A. H., Lee, J., Laiwalla, F., Leung, V., Huang, J., Nurmikko, A., & Song, Y. K. (2020). A scalable and low stress post-CMOS processing technique for implantable microsensors. Micromachines, 11(10), 925.


