Abraham Akinin1,2,*, Jeremy M. Ford1, Jiajia Wu1, Chul Kim1, Hiren D. Thacker2, Patrick P. Mercier1, and Gert Cauwenberghs1.
1 University of California San Diego, La Jolla, California.
2 Nanovision Biosciences, San Diego, California.
* email: akinin1@llnl.gov
Sight is integral to our ability to perceive and interact with the world. The visual system captures information in such detail that it encompasses almost the entire sensory input bandwidth of the brain. And yet, millions of patients are afflicted with blindness requiring assistive technologies and community accommodation. A growing number of these cases are caused by diseases that result neural degeneration of photoreceptor cells in the retina such as Age-related Macular Degeneration. Implantable prosthetics to electrically stimulate the retina and restore vision are an active area of academic research and commercialization efforts [1]. Unfortunately, considerable efforts have not produced a significant quality of life enhancement parallel to the astounding results of cochlear implants to restore hearing. To get there, novel approaches are needed to overcome the field’s main challenges: limited resolution and obtrusive packaging.
To convey the complex images enabling reading and navigation, it is necessary to stimulate (at least) hundreds of retinal ganglion cells in the retina. The conventional clinical approach has been to wirelessly transmit video from a head mounted camera to an implant near the eye which processes the data and generates N current pulses that travel through individual wires to an array of N microelectrodes in contact with the retina. Flexibility and size requirements for such a biocompatible transocular connector has limited the number of channels to 256. Additionally, attempts to scale the conventional system to more pixels are faced with increased power demand for video transmission, decoding, processing, current pulse generation, and charge balancing. The greater power consumption would heat up sensitive tissue to unacceptable levels.
Our proposed solution to scalability, power/heat limitations, and packaging challenges is an optically-addressed nanowire microelectrode array (nMEA) globally controlled by a wireless retinal stimulator system on a chip, RetStim, through a 2-wire flexible connector (Fig. 1A)[2,3]. The pixels of the nMEA are composed of silicon nanowire microphotodiodes. Due to their photosensitivity, the magnitude of the output current at each electrode in the subretinal nMEA will be proportional to the local light intensity[1], illustrated in Fig. 1B. As the system uses the innate visual path, there is no need for wireless video transmission. Although the magnitude of the stimulation is controlled by the incident light, the timing and waveform are globally set by RetStim through a biphasic voltage power pulse.
The inductively powered RetStim system features additional strategies to reduce power consumption and prevent stimulation charge imbalance. Wireless stimulator systems commonly suffer from cascaded power losses due to inductive transmission, rectification, regulation, DC-DC conversion, and stimulator drivers. Thus, any functions carried out by an implant consume more power and dispose the resulting additional heat near sensitive neural tissue. Our design outsources voltage regulation and charge balancing tasks from the implant to the external wearable system that provides power. The external wearable power transmitter directly controls the global shape and maximum amplitude of the stimulation pulses by amplitude modulating a 13.56MHz duty-cycled power carrier wave. When this signal is received by the implant coil, it is rectified and thereby directly provided to the nMEA. This achieves 73% power efficiency in converting the incident RF signal to the desired stimulating current pulses. Moreover, as the system is duty-cycled, there is zero power consumption during inter-pulse intervals. Concurrent with the inductive transmission of pulse power, the implant quantifies globally expended charge by the nMEA during each phase of stimulation and reports it to the external system through load shift keying on the same inductive link. The external system tallies the charge balances it in the following phase of stimulation in order to extend the lifetime of the nMEA and protect surrounding tissues (Fig. 1C). Another notable feature implemented is a calibration system and technique to compensate for power fluctuations that result from the translocation of the inductive receiver coil during natural eye movements [4].
The strategy presented allows for global control of optically-addressed retinal stimulation by combining the RetStim chip with the nMEA. The design overcomes the limitations of transocular interconnect and operates in high power efficiency thanks to externally managed power regulation. The system in biocompatible package [5] (Fig. 1D) features a 1512-channel nMEA, but due to optical addressing and global control, it can be scaled to an arbitrary number of stimulation sites without changing the interconnect or the telemetry system. Ex vivo and in vivo animal studies have demonstrated implantability and functionality [1,6] (Fig. 2.) towards a future human retinal prosthesis which may soon restore vision and improve the quality of life of so many blind patients.
This project is a collaboration between the University of California San Diego and Nanovision
Biosciences.
References
- Ha, S. et al. Towards high-resolution retinal prostheses with direct optical addressing and inductive telemetry. Journal of Neural Engineering. 2016; 13 (5), 056008.
- Akinin, A. et al. An optically-addressed nanowire-based retinal prosthesis with 73% RF-to-stimulation power efficiency and 20nC-to-3μC wireless charge telemetering. IEEE International Solid-State Circuits Conference (ISSCC). 2021; 64, 276-278.
- Akinin, A. et al. An optically addressed nanowire-based retinal prosthesis with wireless stimulation waveform control and charge telemetering. IEEE Journal of Solid-State Circuits. 2021; 56 (11), 3263-3273.
- Akinin, A. et al. Maximizing wireless power transfer to intraocular implants under unconstrained eye movements. IEEE/EMBS Conference on Neural Engineering. 2021; 977-980.
- Liu, YH. et al. Assembly development of a highly flexible and biocompatible optoelectronic neural stimulator for implantable retinal prosthesis. IEEE 71st Electronic Components and Technology Conference (ECTC). 2021; 1538-1543.
- Bosse, B. et al. In vivo photovoltaic performance of a silicon nanowire photodiode–based retinal prosthesis. Investigative Ophthalmology & Visual Science. 2018; 59 (15), 5885-5892.
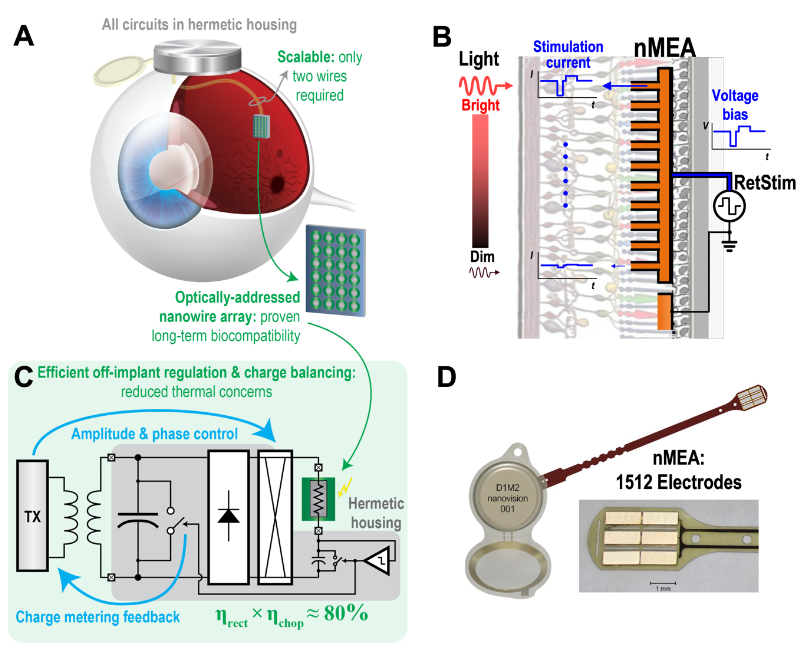
Fig. 1. (A) Advantages of the proposed system based on global control of an optically-addressed electrode array. (B) Operation of the nanowire-based microelectrode array (nMEA) biased by RetStim through a biphasic voltage pulse. (C) Rationale for increased power efficiency and charge monitoring through off-implant modulation of the power waveform. (D) Hermetically packaged and encapsulated retinal prosthesis and photographic detail of 1512 electrode array.
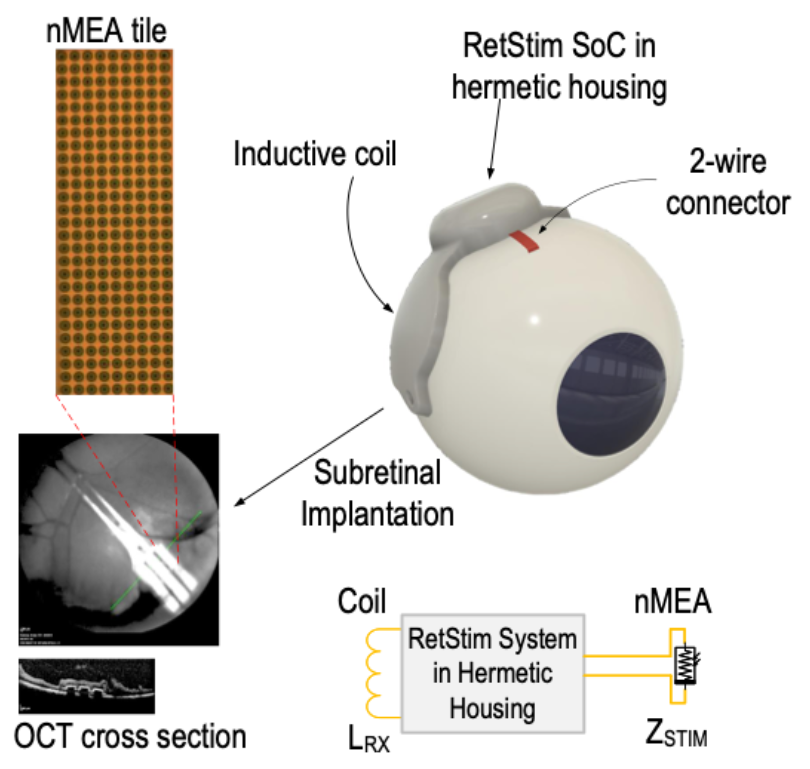
Fig. 2. Overview of the implanted retinal implant with inductive coil attached to the temporal side of the eye. The RetStim chip connects from a hermetic housing to a flexible 2-wire connector to the nMEA. Fundoscopy and OCT imaging of an implanted nMEA in vivo.
Abraham Akinin received the B.S. degree in biomedical engineering and physics from the University of Miami, Coral Gables, FL, USA, in 2010, and the M.S. and Ph.D. degrees in bioengineering from the University of California San Diego, La Jolla, CA, USA, in 2017 and 2020 respectively.He is currently a postdoctoral researcher at the Lawrence Livermore National Laboratory where he is part of the Center for Bioengineering and the Implantable Microsystems Group in the Materials Engineering Division. From 2018 to 2020 he was Bioelectronics Design Engineer at Nanovision Biosciences, developing a scalable and power efficient retinal prosthesis. His research interests include neural prostheses, integrated circuits for medical instrumentation, and implantable or wearable medical devices.
Jeremy Ford received the B.S. degree in Physics from the University of Washington, Seattle, in 2014, and the M.S. degree in bioengineering from the University of California San Diego, La Jolla in 2021. His current research focuses on electronic biomolecule sensing.
Jiajia Wu received the B.S. degree in electronic information engineering from Zhejiang University, Hangzhou, China in 2016, and the M.S. degree in electrical and computer engineering from the University of California San Diego, La Jolla, CA, USA in 2020. She is currently pursuing the Ph.D. degree with UC San Diego.
Her current research interests include miniaturized silicon integrated bioinstrumentation for implantable brain-machine interfaces.
Chul Kim is an assistant professor in the Department of Bio and Brain Engineering and the Program of Brain and Cognitive Engineering at Korea Advanced Institute of Science and Technology (KAIST), Daejeon, South Korea. He received the Ph.D. degree in 2017 from bioengineering, UC San Diego, La Jolla, CA, USA, where he was a postdoctoral fellow from 2017 to 2019. From 2009 to 2012, he was with SK HYNIX, Icheon, South Korea, where he designed power management circuitry for dynamic random-access memory. His current research interests include design of energy-efficient integrated circuits and systems for fully wireless brain-machine interfaces and unobtrusive wearable sensors.
Hiren D. Thacker received the B.S., M.S., and Ph.D. degrees in electrical and computer engineering from the Georgia Institute of Technology, Atlanta, GA, USA, in 2000, 2002, and 2006, respectively. He is currently a Technical Leader in the Technology & Quality organization at Cisco Systems, focused on photonics packaging technology development for next generation optical transceivers. Prior to joining Cisco, Dr. Thacker was Chief Technology Officer at Nanovision Biosciences, Inc. where he directed technical pathfinding and product development of an implantable retinal prosthesis and ultralow light image sensors. Nanovision’s retinal prosthesis was recognized as a Breakthrough Technology by the US FDA. Dr. Thacker was previously a Principal Engineer at Oracle/Sun Microsystems where he helped commercialize the first-ever on-board co-packaged optical transceivers (12x25Gbps/channel) for Oracle’s Infiniband datacenter switching systems. Dr. Thacker has over 35 patents issued or pending and has co-authored over 100 peer-reviewed technical articles.
Patrick P. Mercier received the B.Sc. degree in electrical and computer engineering from the University of Alberta, Edmonton, AB, Canada, in 2006, and the S.M. and Ph.D. degrees in electrical engineering and computer science from the Massachusetts Institute of Technology (MIT), Cambridge, MA, USA, in 2008 and 2012, respectively.
He is currently an Associate Professor in Electrical and Computer Engineering at the University of California San Diego (UCSD), where he is also the co-Director of the Center for Wearable Sensors and the Site Director of the Power Management Integration Center. His research interests include the design of energy-efficient microsystems, focusing on the design of RF circuits, power converters, and sensor interfaces for miniaturized systems and biomedical applications.
Gert Cauwenberghs is Professor of Bioengineering and Co-Director of the Institute for Neural Computation at UC San Diego, La Jolla CA. He received the Ph.D. degree in Electrical Engineering from California Institute of Technology, Pasadena in 1994, and was previously Professor of Electrical and Computer Engineering at Johns Hopkins University, Baltimore, MD, and Visiting Professor of Brain and Cognitive Science at Massachusetts Institute of Technology, Cambridge. He co-founded Cognionics Inc. and chairs its Scientific Advisory Board. His research focuses on micropower biomedical instrumentation, neuron-silicon and brain-machine interfaces, neuromorphic engineering, and adaptive intelligent systems.

