RESEARCH
April 2019
Seeber M., Michel C.
A number of serious diseases, i.e. Parkinson’s, Tourette syndrome and obsessive-compulsive disorders (OCD) are directly associated with subcortical regions. Deep brain stimulation (DBS) is applied as a therapy for severe cases suffering from these diseases. While subcortical areas are known to play an important role in mediating interactions in large-scale networks, it is less clear how their dysfunction affect certain diseases. Furthermore, it is an ongoing discussion how DBS influences subcortical-cortical networks dynamics leading to the treatment’s outcome. In order to study electrophysiological dynamics in subcortical regions, implanted electrodes in these areas are needed. This neurosurgery is naturally only justifiable and possible in the framework of deep brain stimulation therapy, which restricts investigating subcortical dynamics in humans to a few case studies.
In a recent work [1] we addressed the question if subcortical activities can be sensed and reconstructed using non-invasive electroencephalography (EEG), i.e. recordings from electrodes placed on the scalp (Figure 1). This would open up novel possibilities to i) study subcortical dynamics in a much larger cohort in humans and ii) investigate subcortical and cortical interactions and their network dynamics.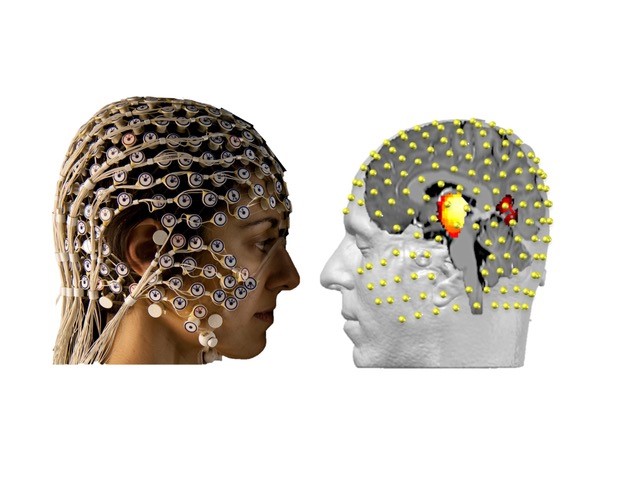 Fig.1 High-density EEG recordings in combination with source reconstruction techniques are able to detect subcortical electrophysiological activities in humans. Individual magnetic resonance scans (T1) are used to construct head models describing the electrical field propagation through different tissues from every point in the brain to all electrodes at the scalp. Based on these electrical relationships and physiological constraints, it is possible to reconstruct cortical and subcortical dynamics using inverse methods (LAURA, i.e. L2 norm based).
Fig.1 High-density EEG recordings in combination with source reconstruction techniques are able to detect subcortical electrophysiological activities in humans. Individual magnetic resonance scans (T1) are used to construct head models describing the electrical field propagation through different tissues from every point in the brain to all electrodes at the scalp. Based on these electrical relationships and physiological constraints, it is possible to reconstruct cortical and subcortical dynamics using inverse methods (LAURA, i.e. L2 norm based).
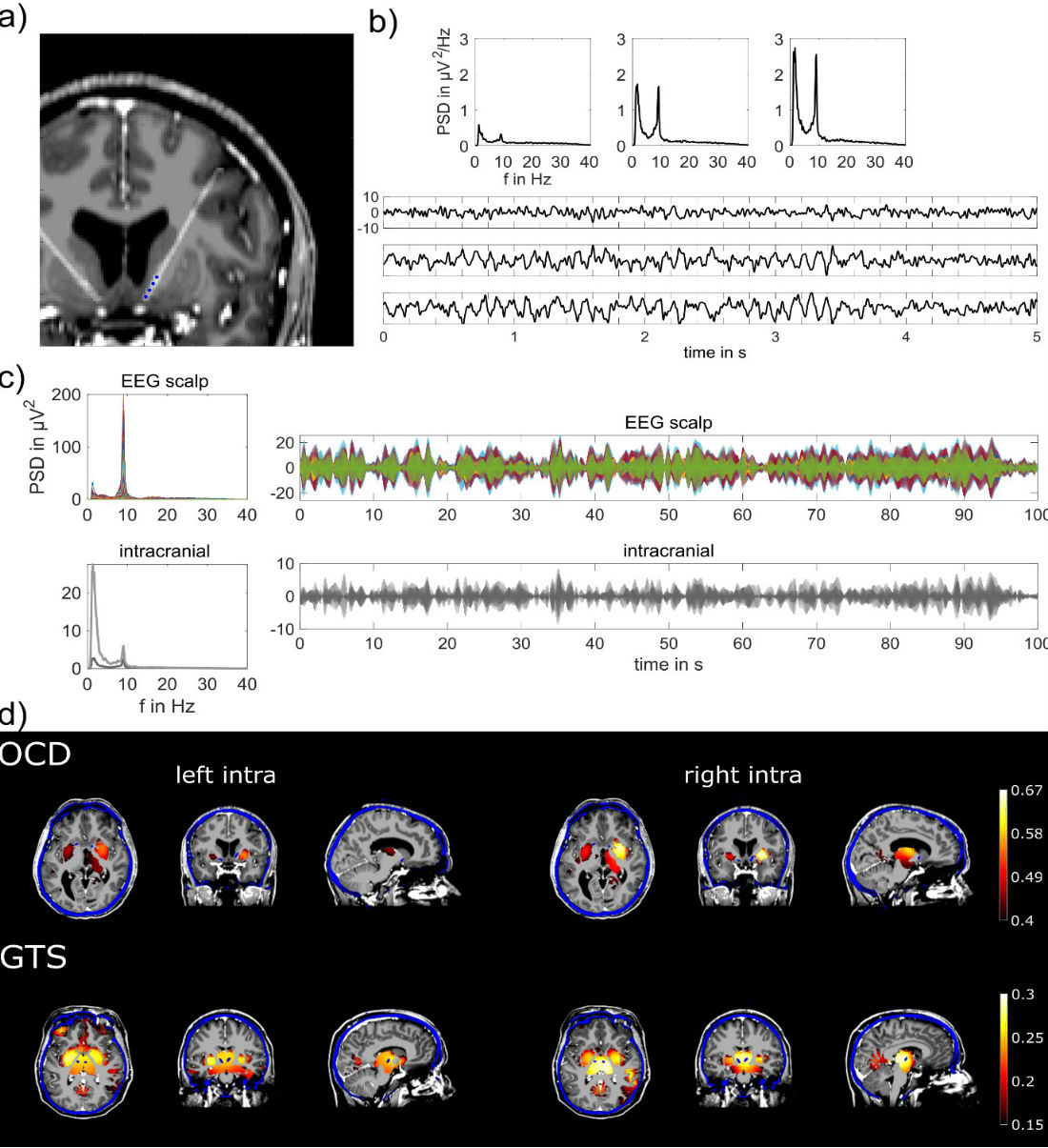
In our study, we recorded high-density EEG (256 channel) simultaneously with intracranial electrodes placed in subcortical regions. Externalization of DBS electrodes in two patients suffering from Gilles de Tourette Syndrome (GTS) and two patients from obsessive-compulsive disorder (OCD) undergoing electrode implantation in the thalamus and the nucleus accumbens (Figure 2a), respectively, provided the unique opportunity to record subcortical activities from intracranial electrodes simultaneously with scalp EEG. These recordings enable direct comparison between the ground true subcortical dynamics with reconstructed estimates of these activities derived from scalp EEG. Indeed, we found significant correlations between subcortical signals and EEG source reconstructed signals in regions proximate to the actual implantation sites.
In detail, we focused on the analyses of alpha fluctuation during bedside recordings while patients were at rest for 5 minutes with eyes closes. In every patient, we observed a prevalent alpha peak in the frequency range of 8-10 Hz in their EEG scalp recordings, which is one of the most prominent EEG phenomena. Interestingly, we found identical frequency peaks in the power spectral densities (PSD) of the intracranial recordings (Figure 2b/c). Based on these findings we compared alpha amplitude time series derived from intracranial recordings with non-invasively reconstructed estimates computed from scalp EEG. EEG source reconstruction is based on individual head models, describing the electrical field propagation from every point in the brain to the scalp electrodes’ locations [2-5]. Inverting these relationships using physiological constraints, i.e. spatial smoothness [6], for solving the ill-posed inverse problem enables to reconstruct cortical and, as shown in our recent work [1], subcortical dynamics.
Fig. 2 a) MRI overlaid with post-op CT scan in an OCD patient, showing the intracranial DBS electrodes locations indicated as blue dots, i.e. the nucleus accumbens. b) PSD and exemplary time courses are displayed from three bipolar derivations at the top, middle and lowest pair of the four intracranial electrode contacts. c) PSD of scalp EEG and intracranial recordings were used to select individual alpha peak frequency (left panel). Exemplary time courses of alpha envelopes showing similarities and differences of scalp and intracranial signals (right panel). Different colors are displaying different recording electrodes; light/dark grey colors correspond to left/right hemispheric implantation sites. All time courses are scaled in µV. d) EEG source images illustrating significant correlation between source reconstructed and actually recorded alpha envelopes in subcortical areas. Intracranial electrodes are implanted in the nucleus accumbens (OCD) and in the centromedial thalamus (GTS) of the left and right hemisphere. Accordingly, highest correlations between source reconstructed and intracranial signals are located in target, i.e. implantation regions or in close proximity to it. MRI images (greyscale) are overlaid with post-op CT (blue) scans and EEG source imaging results (warm colors). Intracranial electrode positions are visible as blue dots and were used to select the view of these images.
EEG source reconstruction results in time series at virtual points covering the whole brain’s grey matter volume. Individual head models including grey matter volume segmentation are derived from magnetic resonance images (MRI). Therefore, EEG source reconstruction combines and associates functional (EEG) with structural (MRI) data. In order to assess similarity of reconstructed and actually recorded subcortical signals, we used correlation and non-parametric statistical tests [7, 8]. In fact, these analyses showed that reconstructed signals are significantly correlated (p ≤ 0.01 corrected, permutation test) with actually recorded subcortical signals. These analyses result in correlation values for all virtual points in the grey matter, which enables spatial comparisons with the actual signal origin given by the DBS electrode positions. These positions were gained from post-operative computed tomography scans, which were spatially aligned to MRI space used for EEG source reconstruction [9, 10]. The Euclidian distance between actual subcortical signals and the maximal correlated estimated signal derived from EEG source reconstruction are ranging between 14.8 and 23.5 millimeter. These distances are in the range of previously reported uncertainties of source reconstruction techniques [11, 12]. Yet, there is quite some room for improvement in respect to source reconstruction methodology.
In summary, we report that non-invasive EEG recordings contain subcortical activities, which can be reconstructed using source reconstruction. This opens up novel possibilities to study subcortical activities in humans in a larger cohort. In our future and ongoing research, we aim to investigate subcortical and cortical network dynamics fully non-invasive. Deeper understanding of these interactions and their association to specific diseases might lead to improvement of clinical treatments.
About the Authors
 Martin Seeber studied Electrical and Biomedical Engineering at the Graz University of Technology and received his PhD in 2017 from the Laboratory of Brain-Computer Interfaces. Afterwards he joined the Fuctional Brain Mapping Lab of Christoph Michel at the University of Geneva. He currently is working as postdoctoral researcher focusing on analyses and research of spontaneous EEG dynamics.
Martin Seeber studied Electrical and Biomedical Engineering at the Graz University of Technology and received his PhD in 2017 from the Laboratory of Brain-Computer Interfaces. Afterwards he joined the Fuctional Brain Mapping Lab of Christoph Michel at the University of Geneva. He currently is working as postdoctoral researcher focusing on analyses and research of spontaneous EEG dynamics.
 Christoph Michel has studied Behavioral Biology at the Swiss Federal Institute of Technology (ETH) in Zurich. He is now Full Professor at the Neuroscience Department of the Medical Faculty of the University Geneva and Director of the EEG core of the Biomedical Imaging Center Lausanne-Geneva. He is the Past-President of the Swiss Society for Neuroscience. He has authored over 260 articles and book chapters and is first author of the book “Electrical Neuroimaging” published by Cambridge University Press in 2009. He is Editor-in-Chief of the journal Brain Topography, Associate Editor of the European Journal of Neuroscience, and Associate Editor of Frontiers in Behavioral Neuroscience. His group is applying electrical neuroimaging tools to cognitive as well as clinical studies with the aim to unravel the spatio-temporal properties of large-scale brain network communication in health and disease.
Christoph Michel has studied Behavioral Biology at the Swiss Federal Institute of Technology (ETH) in Zurich. He is now Full Professor at the Neuroscience Department of the Medical Faculty of the University Geneva and Director of the EEG core of the Biomedical Imaging Center Lausanne-Geneva. He is the Past-President of the Swiss Society for Neuroscience. He has authored over 260 articles and book chapters and is first author of the book “Electrical Neuroimaging” published by Cambridge University Press in 2009. He is Editor-in-Chief of the journal Brain Topography, Associate Editor of the European Journal of Neuroscience, and Associate Editor of Frontiers in Behavioral Neuroscience. His group is applying electrical neuroimaging tools to cognitive as well as clinical studies with the aim to unravel the spatio-temporal properties of large-scale brain network communication in health and disease.