RESEARCH
Joel Libove, David Schriebman and Mike Ingle
Furaxa, Inc., Berkeley, CA, USA
Ultrawideband (UWB) microwave pulses having widths of 20-50 picoseconds can penetrate the skull and travel into deep brain tissues. Recently developed radar integrated circuits (ICs) can generate customizable pulses, launch them into the cortex, and monitor the resulting reflections from brain tissue boundaries. The amplitude of these reflections varies slightly, in real time, due to metabolic changes in brain tissue undergoing localized activity, enabling functional activity to be spatially mapped. The arrival time of these reflections also varies with the pulsation of arterial walls, additionally facilitating real-time imaging of neurovascular structures. A helmet under development, with 128 dual-channel radar ICs shows promise for enabling a wearable brain machine interface (BMI).
Existing Functional Imaging Methods
Functional magnetic resonance imaging (fMRI) has evolved to where rudimentary “thought identification” is being achieved [1], [2], [3]. However, subjects must lie inside an MRI machine, precluding its use in operational environments. Functional near infrared (fNIR) imaging devices are semi-portable, and detect activity in shallow cortical gyri, but the infrared radiation cannot penetrate deeper tissues [4], [5], [6]. Electroencephalography (EEG), while portable, has limited spatial resolution. Magnetoencephalography (MEG) has slightly better spatial resolution, and a promising semi-portable version has recently been demonstrated [7], although it must be used in a non-portable enclosure providing cancellation of the earth’s magnetic field. Hence, the need remains for an imaging technology with improved portability, spatial and temporal resolution, and imaging depth.
Evolution of Microwave Functional Imaging
While microwave energy has been used for over a decade for structural imaging, its use for extracting functional neural information was first shown in 2014, when Li et al [8] transmitted a signal through the head of a rodent and measured changes in phase and attenuation. Microwave measurement of brain activity in a human [9] was demonstrated in 2015. Microwave reflectivity changes over a portion of the left primary motor center (M1) were seen in response to the subject pressing a button with his right forefinger. These experiments used a single antenna and a 0.3V pulse, so the signal-to-noise ratio (SNR) was poor. However, these results were sufficiently encouraging to spur the current development of the improved ICs and helmet architecture described herein.
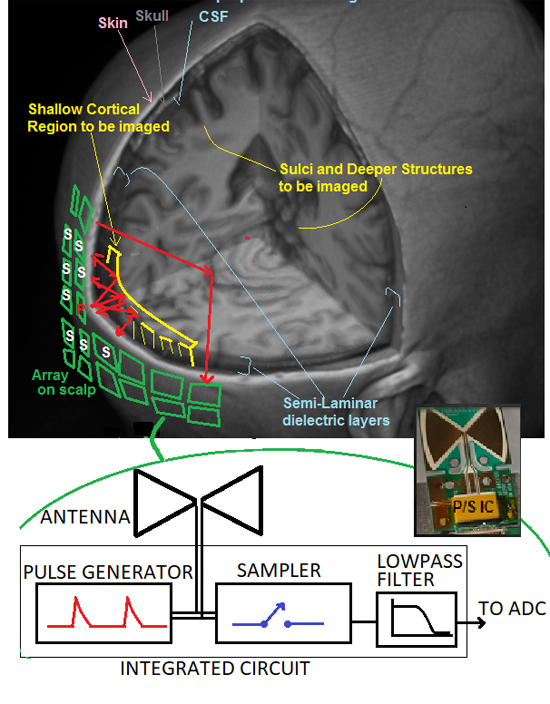
Figure 1. Proposed array of radar assemblies, each containing a pulse generator, antenna and sampler. One assembly at a time launches a pulse stream into the head while samplers on nearby assemblies receive reflected signals from cortical gyri and other structures. A first-generation IC/antenna assembly is shown at lower right.
Basic Microwave Brain Imager Architecture
Microwave imaging systems generally consist of an array of transmit/receive assemblies that emit either frequency-swept sine waves, or UWB pulse trains. A typical system, such as one under development by Furaxa, Inc., consists of a hat with an array of 128-256 single-IC radars over the scalp, as illustrated in Figure 1, each radar transmitting 20-100 million pulses/second, via a 1 x 1 cm antenna, while it, and its neighboring units receive resulting echoes as they reflect off dielectric boundaries. Each echo, whose arrival time is roughly proportional to the total path length from the transmitting antenna, to the tissue boundary, and back to the receiving antenna, is coupled into the sampler, whose output is fed to an A/D converter (ADC). At these boundaries, small changes in dielectric properties due to functionally-induced real-time changes in metabolic activity cause these echo signals to vary, very slightly, in amplitude.
The sampler and pulser in these radar elements each use a Dynamic Cascode Exchange (DCE) sampler/pulser circuit topology [10-16] that can generate pulses of selectable amplitude and width as well as dynamically alternating polarity. Narrow pulses allow fine spatial resolution, while wide pulses contain more low frequency content and can penetrate more deeply, at the expense of reduced resolution.
Figure 2 (top portion) shows the principle by which functional activity changes the position and amplitude of reflected energy from functional and vascular boundaries. The arrival time of echoes from layers of tissue is proportional to tissue boundary depth. Reflections R1 and R2 are from static structural boundaries, such as skull and CSF, and do not vary with time, while R3 and R4 are from functional boundaries (brain regions whose dielectric properties vary with activity), and the variation in their amplitude indicates functional change [9]. Changes of echo amplitude, displayed in vertical chart-recorder format, indicate changes due to functional activity or motion of blood vessels. The bottom of figure 2 shows experimentally acquired microwave echo stream from the left occipital region of subject. The chart scrolls vertically, with 1-second intervals shown. Pulsation of an artery in the subarachnoid space (green trace toward left) shows a familiar pulse pressure waveform, while waveforms from deeper regions (further right traces) show more rounded shape, possibly due to CSF pulsation in the lateral ventricle. Probable neural activity (labeled red and green traces) are from echoes with arrival time corresponding to depth of a cortical gyrus.
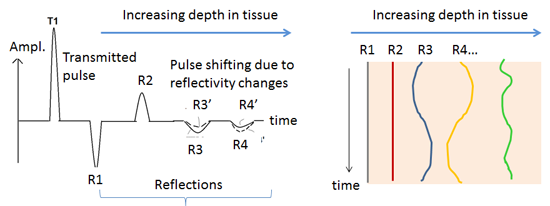
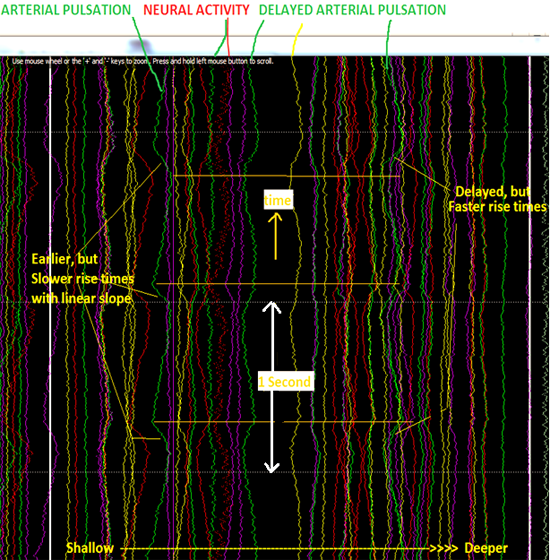
Figure 2. Top left: Arrival times of echoes from tissue boundaries are proportional to boundary depths. Echo amplitude changes, in chart-recorder format (Top right and Bottom), indicate changes due to functional activity and blood vessel and tissue motion. Bottom: Echo stream from left occipital region of subject. Pulsation of artery in subarachnoid space (green trace toward left) shows sharp pulse pressure waveform. Probable neural activity (labeled red and green traces) are from echoes with arrival time corresponding to depth of a cortical gyrus.
Scattering Mitigation and SNR Improvement Strategies
The ability to do usable functional and vascular imaging is hampered by poor SNR, as well as severe spatial blurring due to broad antenna radiation patterns and scattering in tissues. Conventional beam steering algorithms used for radar in air work poorly in brain tissue, in which radio waves are scattered. We are exploring machine learning algorithms, in collaboration with the Gallant Laboratory at University of California, Berkeley, with the goal of mitigating the effects of scattering. We further believe the SNR limitations will be effectively addressed by new ICs under development that can be integrated into synchronized radars that can be densely packed on the surface of the scalp. A 43dB total SNR improvement is expected, using the following strategies:
- Increasing transmit pulse voltage from 0.3V to 2.5V, potentially increasing SNR by factor of 8 (18dB)
- Using streams of alternating polarity pulses, and subtracting responses from these respective opposing pulses, thereby cancelling baseline drift and 1/f noise. Summed signal amplitude will be 2 x the response from each pulse, while baseline drift and low frequency noise will largely be cancelled, enabling the ICs to better detect the very faint changes in reflectivity due to neural and neurovascular activity.
- Collecting echoes using samplers on neighboring antennas, not simply the sampler shared by the transmit antenna. Electromagnetic simulation results suggest that, for a 1cm antenna pitch, this will result in a 13dB SNR improvement.
- Concurrently imaging approximately 18 spatially separate domains, thereby increasing signal collection efficiency. These domains are effectively isolated, and do not interfere with one another, due to inter-domain attenuation and the use of time gating to reject signals from non-local domains, which inherently have later arrival times. Simulation results indicate a 12dB potential SNR improvement.
Conclusion
While much work remains before a usable UWB BMI is achieved, progress is being made. If successful, the functional imaging technology described herein could accelerate neuroscience research, and potentially enable networked BMIs, and neuroprosthetic devices. The low amount of energy employed (<30mW total average power) may further render this modality safer than existing active imaging modalities such as fMRI.
Acknowledgements
We gratefully acknowledge valuable ongoing collaborations with the Gallant Laboratory at UC Berkeley.
References
- Naselaris, T.,Prenger, R. J., Kay, K. N., Oliver, M. & Gallant, J. L. “Bayesian reconstruction of natural images from human brain activity”. Neuron 63, 902–915 (2009)
- Huth, A. G.,Nishimoto, S., Vu, A. T. & Gallant, J. L. “A continuous semantic space describes the representation of thousands of object and action categories across the human brain”. Neuron 76, 1210–1224 (2012)
- G.Huth, W.A. de Heer, T.L. Griffiths, F.E. Theunissen, J. L. Gallant, “Natura Speech Reveals the Semantic Maps that Tile Human Cerebral Cortex” Nature 532 453-458 (28 April 2016)
- Ferrari, V. Quaresima, “A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application”,Neuroimage,2012 Nov 1;63(2):921-35. doi: 10.1016/j.
- Eggebrecht, S. Ferradal, A. Robichaux-Viehoever, M. Hassanpou, H. Dehghani, A. Snyder, T. Hershey and J. Culver, “Mapping distributed brain function and networks with diffuse optical tomography”, Nature Photonics, May 18, 2014. DOI: 10.1038/Nphoton.2014.107.
- Balconi and M. E. Vanutelli. Hemodynamic (fNIRS) and EEG (N200) correlates of emotional inter-species interactions modulated by visual and auditory stimulation.Sci. Rep. 6, 23083; doi: 10.1038/srep23083 (2016).
- Boto, N. Holmes, J. Leggett, G. Roberts, V. Shah, S. Meyer, L. Muñoz, K. Mullinger, T. Tierney, S. Bestmann, G. Barnes, R. Bowtell, M. Brookes, “Moving magnetoencephalography towards real-world applications with a wearable system”, Nature 2018/03/21/online, Volume 555 p.657
- Li, X.P.et al. “The dynamic dielectric at a brain functional site and an EM wave approach to functional brain imaging”, Rep.4, 6893; DOI:10.1038/srep06893 (2014).
- Libove, D. Schriebman, M. Ingle, and B. Wahl, “Wearable brain imager/BMI technology for structural, vascular and functional extraction,” in Proc. IEEE International Conference on Systems, Man, and Cybernetics (SMC 2016), Budapest, Hungary, October 2016, pp. 3806-3811.
- M. Libove and S.J. Chacko, “Methods, apparatuses and systems for sampling or pulse generation”, US Patent 6,433,720, Aug. 13, 2002.
- M. Libove and S.J. Chacko, “Methods and apparatuses for multiple sampling and multiple pulse generation”, US Patent 6,642,878, November 4, 2003.
- M. Libove, B.R. Illingworth, S.J.Chacko, H.L. Levitt, “Monolithic sampler/pulser exceeds 100 GHz”, Microwave Journal, August 2008, pp 86-105.
- Libove, “Potential Application for Combined Microwave Pulser/sampler ICs in Functional Neuroimaging”, IEEE MTT-SCV, Mar. 12, 2015, Santa Clara, CA.
- Libove, “New Microwave Sampler/Pulser ICs Enable Real-Time Functional Neuroimaging Prototype”, IEEE, AP-S/MTT-S Denver Chapter Meeting, May 28, 2015, Boulder, CO
- Libove, M. Ingle and D Schriebman, “Method and apparatus for non-invasive real-time biomedical imaging of neural and vascular activity”, Patent Pending 14/885,236, October 16, 2015.
- Libove, “Picosecond Pulse Imaging – Promising but Challenging Modality for Wearable Functional and Structural Brain Imaging”, IEEE, EMBS-SCV Meeting, May 17, 2017, Stanford University Medical School, Palo Alto, CA.
About the Author:
 Dr. Joel Libove (M) is president of Furaxa, and Chairman of Ultraview Corp. Specializing in analog/microwave circuit design, he has 15 patents, and received two industry awards for developing the first real-time computer bus protocol violation recognition/triggering system. He has designed over 200 products, including disk controllers, VME and PCI bus analyzers, and DC-4GSPS A/D boards. Joel also designed 36 high dynamic range variable-width DC-150 GHz pulse generation and sampling ICs in GaN, InP, SiGe and CMOS/SOI, with which he successfully demonstrated vascular and functional brain imager prototypes. He received a BSEE from Cornell, and MS and Ph.D. in EECS from UC Berkeley in 1978 and 1981.
Dr. Joel Libove (M) is president of Furaxa, and Chairman of Ultraview Corp. Specializing in analog/microwave circuit design, he has 15 patents, and received two industry awards for developing the first real-time computer bus protocol violation recognition/triggering system. He has designed over 200 products, including disk controllers, VME and PCI bus analyzers, and DC-4GSPS A/D boards. Joel also designed 36 high dynamic range variable-width DC-150 GHz pulse generation and sampling ICs in GaN, InP, SiGe and CMOS/SOI, with which he successfully demonstrated vascular and functional brain imager prototypes. He received a BSEE from Cornell, and MS and Ph.D. in EECS from UC Berkeley in 1978 and 1981.